Findings seen despite negligible remaining lebrikizumab serum concentrations following treatment withdrawal
By Lori Solomon HealthDay Reporter
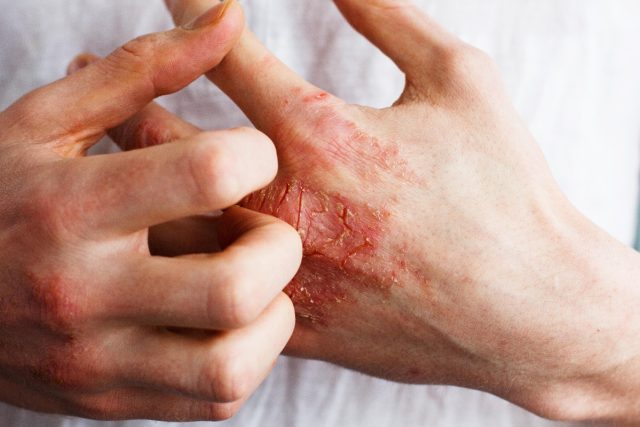
TUESDAY, June 18, 2024 (HealthDay News) — Lebrikizumab is associated with sustained effects for moderate-to-severe atopic dermatitis for up to week 52 following withdrawal of treatment, according to a study presented at the annual Revolutionizing Atopic Dermatitis Conference, held from June 8 to 10 in Chicago.
Jonathan I. Silverberg, M.D., Ph.D., M.P.H., from the George Washington University School of Medicine and Health Sciences in Washington, D.C., and colleagues examined the relationship between lebrikizumab serum concentration levels and sustained clinical response after treatment cessation among lebrikizumab responders who discontinued treatment.
The researchers found that 17 of 60 lebrikizumab responders who were withdrawn from treatment (28 percent) maintained an Eczema Area and Severity Index >90 percent (EASI 90) for 80 percent of the visits during the 38-week withdrawal period. A similar percent achieved EASI 90 at week 52 and did not use rescue medication. At week 16, the mean serum lebrikizumab concentration was 92.4 μg/mL, and the mean serum concentrations decreased to 7.3 μg/mL at week 32 and 0.15 μg/mL at week 52 (92 and >99 percent reduction, respectively). Twelve of 16 patients had serum concentrations below the lower level of quantification (0.09 μg/mL) for the clinical assay at week 52. The mean elimination half-life for lebrikizumab was approximately 24.5 days.
“Further studies are needed to identify and characterize this subpopulation of atopic dermatitis patients and lebrikizumab’s potential disease-modifying properties,” the authors write.
Several authors disclosed financial ties to pharmaceutical companies, including Eli Lilly, which manufactures lebrikizumab and funded the study.
Copyright © 2024 HealthDay. All rights reserved.