Approval is for new, first-line biologic treatment of eczema that is not well controlled with topicals in people 12 years and older
By Lori Solomon HealthDay Reporter
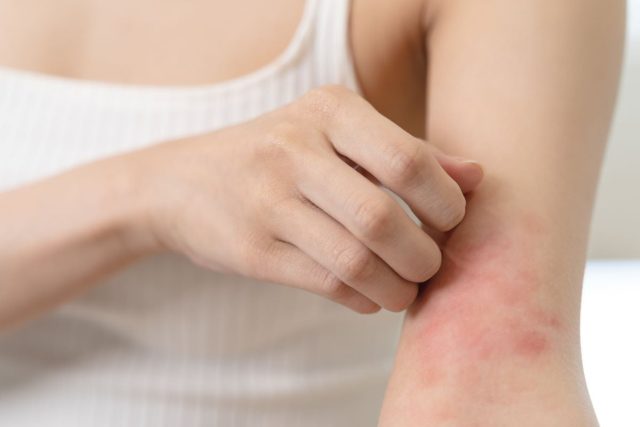
WEDNESDAY, Sept. 18, 2024 (HealthDay News) — The U.S. Food and Drug Administration has approved Ebglyss (lebrikizumab-lbkz) for adults and children aged 12 years and older with moderate-to-severe atopic dermatitis.
The targeted interleukin-13 inhibitor is administered via a 250-mg/2 mL injection with or without topical corticosteroids in patients with atopic dermatitis not well controlled despite treatment with topical prescription therapies. The approval is dosed as a single monthly maintenance injection (250 mg) following the initial phase of treatment of 500 mg (two 250-mg injections) at week 0 and week 2, followed by 250 mg every two weeks until week 16 or later when adequate clinical response is achieved.
The approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, with >1,000 participants. In an average of two studies (ADvocate 1 and 2), 38 percent of those who took Ebglyss achieved clear or almost-clear skin at 16 weeks versus 12 percent with placebo. Ten percent of individuals saw these results as early as four weeks. Among participants achieving clear or almost-clear skin at week 16, 77 percent maintained those results at one year with once-monthly dosing. Furthermore, 48 percent of responders who were switched from Ebglyss to placebo at week 16 maintained results at one year. Participants in both studies showed significant itch relief with Ebglyss (43 percent at 16 weeks versus 12 percent who took placebo).
“Patients still struggle to control their moderate-to-severe atopic dermatitis with currently available therapies,” Jonathan Silverberg, M.D., Ph.D., from George Washington University in Washington, D.C., and first author of a paper published in the New England Journal of Medicine summarizing Ebglyss clinical trials, said in a statement. “Many experience poor long-term disease control, and severe itch can significantly impact their daily lives.”
Approval of Ebglyss was granted to Eli Lilly.
Copyright © 2024 HealthDay. All rights reserved.